U-Tox Certified Training
This certified training program is intended to provide drug test administrators information to better understand the U-Tox™ product and testing procedure. Please read through all of the information within this training program. After completing the training portion, you may test your knowledge with our online certification quiz. Once you have successfully passed the quiz with 100% accuracy, you will receive a personalized certificate of training which you can print for your records.

Product Overview
The U-Tox is a preliminary screening test for the presence of drugs. The U-Tox is a qualitative, immunoassay urine-based rapid test that incorporates collection of a urine specimen and the testing of multiple drugs simultaneously. For a quantitative result or to confirm a presumptive positive screen, a second, alternative chemical method should be performed on the same urine sample. Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS) or Gas Chromatography/Mass Spectrometry (GC/MS) are the preferred confirmation methods.
Product Features And Benefits
- Exclusive panel configurations including relevant drugs of abuse
- Customizable configurations available
- EtG panel configurations available Integrated (all in one cup)
- Fast results in minutes
- 60 minute result stability
- Strong line intensity for easy to read results
- Leak proof lid
- Built-in SVT (Specimen Validity Tests) available
- Highly accurate, sensitive, and specific
- Unique QR (Quick response code) ties the device to the donor
- Clear, flat front panel design all on one side for quick Interpretation
- Large opening provides an easier specimen collection
- Easy to use, single step procedure
- No minimum fill requirement
Storing And Packaging
- Store tests at room temperature
- Begin by checking the foil pouch to ensure that it has not been compromised and the lot number and expiration date are valid.
- Do not use past the expiration date
(Example: 2018-6 = Valid through June 30, 2018) - Minimum order is one case (25 devices)
Collecting The Sample
-
Tear open the pouch and remove cup and lid.
-
Request the donor to void into the cup.
-
Once the sample has been collected, replace lid and tighten firmly to ensure urine will not leak.
-
Place the device on a flat surface while the test is running and the results are read.

Temperature Strip Validation
-
Prior to reading the test results, turn the U-Tox around to the back and read the temperature strip to make sure the specimen is within normal range.
-
The temp should be within 90-100°F. (Indicated by the green dot)

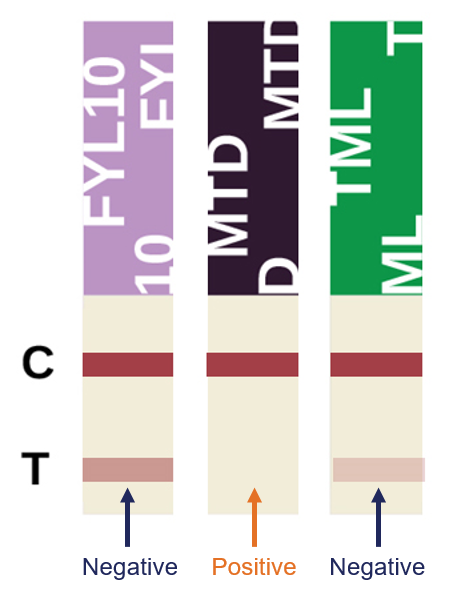
Result Interpretation
Some of the U-Tox test strips will contain two drugs. Strips with two drugs will be arranged on the left side of the cup, followed by individual drug test strips, then EtG and SVT (if included). The control Line (C) is the uppermost line appearing on each strip. Before reading the test result Lines, verify that all control Lines have formed. Otherwise, the test is Invalid and the test results must not be used.
A Negative result is indicated by the presence of a reddish-purple control line and the presence of a corresponding reddish-purple test line.
A Negative result can be read as soon as both the control and test lines have formed. Wait the full 5 minutes before reading a presumptive positive result. Results are stable up to 60 minutes.
A Positive result is indicated by a reddish-purple control line and the absence of the corresponding test line for any particular drug within the testing panel.
The intensity of the test Lines may vary. Any line, without regard to intensity, color or size, indicates a Negative result for that drug.
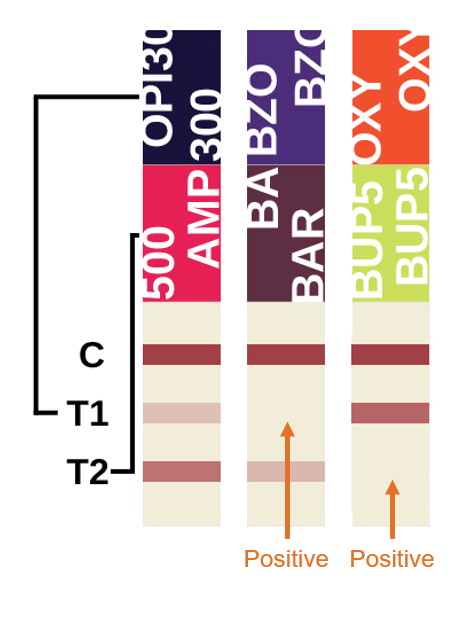
Multi-Drug Interpretation
The multi-drug strips consist of the top control line and two test regions (labeled T1 and T2 below) representing each of the multi-drugs.
Within This Diagram:
T1 corresponds with: OPI, BZO, OXY test results
T2 corresponds with: AMP, BAR, BUP test results
This diagram shows a positive result for BZO and BUP and a negative result for OPI, AMP, BAR and OXY
Test Your Knowledge By Taking The Certification Quiz
You have now completed the U-Tox training. You may go back over any or all of the material above anytime. Once you feel confident with the material presented, you may proceed to the certification quiz by clicking the button below. After you complete the quiz with 100% accuracy, a certificate will be generated on your screen that you may print for your records.
